inversion of sucrose by polarimeter|sucrose inversion research paper : exporters In this practice, the rate constant of sugar inversion should be determined by the methods described above. The optical rotation of the solutions is measured with a polarimeter at a . Resultado da Founded in 1835. Revenue: $10+ billion (USD) Wholesale. Competitors: Unknown. Celesio likes being a middleman when it comes to pharmaceuticals. The company is one of Europe's largest drug wholesalers, holding market-leading positions in several of the countries it serves. Its .
{plog:ftitle_list}
Resultado da Resolva provas do concurso CONPASS, veja o edital do ano. Faça simulado online com as questões da prova do concurso.
The rate of the reaction in which sucrose is hydrolyzed to glucose and fructose is determined by observing the change in the optical rotation of sucrose as the reaction proceeds.You will be using a Vernier Go Direct Polarimeter with a 589 nm light source to measure the angle of rotation of polarized light passing through solutions of sucrose in water. You will .1. Measure α with polarimeter. But all 3 sugars are optically active, so α doesn’t vanish when S is 100% converted. 2. Rather, α = A exp(–k eff t) + B (exponential + background) 3. t = 0: α (≡ α .In this practice, the rate constant of sugar inversion should be determined by the methods described above. The optical rotation of the solutions is measured with a polarimeter at a .
sucrose inversion research paper
sucrose inversion rate pdf
In this experiment, the extent of sucrose hydrolysis in the presence of a strong acid was followed through polarimetry. As the reaction reached completion, the angle of rotation for plane polarized light slowly inverted from 6° to -2°.Rinse the polarimeter cell several times with distilled water, and determine its volume. The reaction is initiated by mixing the solutions (the sucrose solution plus one of the acid .Apparatus for polarimetric measurement of the inversion of sucrose into glucose and fructose. Preparation. Read up on the basics of reaction kinetics in the literature (e.g. Chapter 9 of the .
In this experiment, hydrochloric acid is used to catalyze the reaction while its rate is monitored using a polarimeter. The experiment will be repeated using the enzyme invertase to catalyze the reaction.
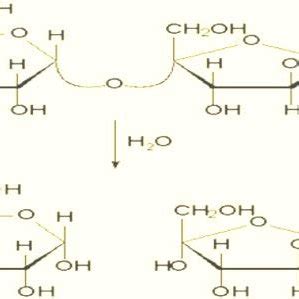
Sucrose inversion occurs when it is dissolved in water in the presence of a catalyst such as an acid or an enzyme. Sucrose is broken into glucose and fructose, two other sugars. Because . Sucrose inversion (Fig. 1) occurs when the glycosidic linkage of the disaccharide is hydrolyzed, releasing the monosaccharide units. The term inversion is derived from observing .The rate of hydrolysis or ‘inversion’ of sucrose, by polarimetry. Aim: Determination of the rate at which sucrose hydrolyses by calculating its angle of optical rotation using a polarimeter at 25°C in 15 minute intervals for a period .
work hard on the test quotes
rate constant of sucrose inversion
work instruction for rockwell hardness tester
Inversion of Sucrose 1 Purpose: The rate of reaction between sucrose and water catalyzed by hydrogen ion is followed by measuring the angle of rotation of polarized light passing through the solution. Non-linear . Polarimeter (described in the Appendix); sodium-vapor lamp; thermostat and The polarimeter was set up. The stopwatch was started and both solutions A and C were mixed thoroughly by pouring back and forth a few times. As quickly as possible, a clean, dry polarimeter tube was filled with the mixed solution and the angle of rotation was measured and recorded. . From graph 1 and 2, the rate of inversion of sucrose was .He also discovered that other compounds i.e., turpentine and sucrose solutions were capable of rotating the light. He attributed this "optical activity" to certain features in their molecular structure (asymmetry). Based on his research, he designed one of the first polariscopes, and formulated the basic quantitative laws of polarimetry. In .Kinetics of the Inversion of Sucrose J0603 Objectives/Goals Our goal was to use polarimetry to measure the activation energy of the inversion of sucrose in the presence of a catalyst. Sucrose inversion occurs when it is dissolved in water in the presence of a catalyst such as an acid or an enzyme.
0:07 Kinetics of Sucrose Inversion0:30 Preparing the reactions1:15 Polarimeter and the inversion reaction2:45 Measuring and mixing reactants6:30 Using polari.
The time dependence of the optical rotation of a sucrose solution upon adding HCl (protons) shows that the optical rotation slowly changes from +66.3 o to –36.5 o. The sign change is due to the fact that the optical rotation of fructose is oposite to and greater than that of glucose.In this practice, the rate constant of sugar inversion should be determined by the methods described above. The optical rotation of the solutions is measured with a polarimeter at a constant acid concentration. A description of the polarimeter and instructions for use can be found together with the equipments. For moreThe Inversion of Sucrose Introduction This experiment is the second in a series of kinetic studies. Sucrose hydrolyzes in acidic . Rinse the polarimeter cell several times with distilled water, and determine its volume. The reaction is initiated by mixing the solutions (the sucrose solution plus one of the acid solutions) in a beaker, then .
A polarimeter [1] is a scientific instrument used to measure optical rotation: the angle of rotation caused by passing linearly polarized light through an optically active substance. [ 2 ] Some chemical substances are optically active, and linearly polarized (uni-directional) light will rotate either to the left (counter-clockwise) or right .3. Refer to the accompanying figure of three connected Hg manometers. If atmospheric pressure is 754 torr, h1 = 111 mm, h2 = 83 mm, h3 = 192 mm, and h4 = 289 mm, what are the pressures P1 and P2 (in Torr)? a. 865 & 948 b. Sucrose or table sugar is obtained from sugar cane or sugar beets. Sucrose is made from glucose and fructose units. The glucose and fructose units are joined by an acetal oxygen bridge in the alpha orientation. The structure is easy to recognize because it contains the six member ring of glucose and the five member ring of fructose.
polarometer for sugar inversion
Dense inverted sugar syrup (Trimoline) Inverted sugar syrup, also called invert syrup, invert sugar, [1] simple syrup, sugar syrup, sugar water, bar syrup, syrup USP, or sucrose inversion, is a syrup mixture of the monosaccharides glucose and fructose, that is made by hydrolytic saccharification of the disaccharide sucrose.This mixture's optical rotation is opposite to that .
We would like to show you a description here but the site won’t allow us.
The measurements are carried out in a polarimeter, which essentially consists of a sodium lamp, a polarizer, a cuvette and an analyzer. . The progress of the sucrose inversion reaction can be easily followed because both the sucrose sugar and the .
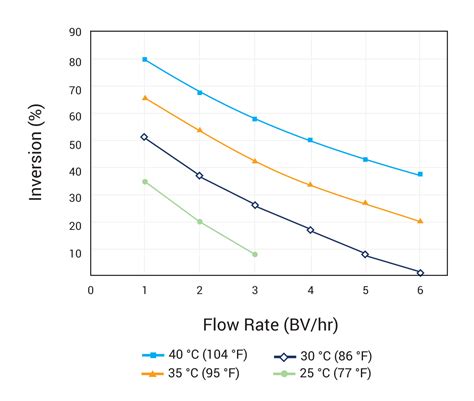
Polarimetry, using a flow-through polarimeter at 589 nm, is the general method of sucrose analysis in bulk raw and white sugars. Traditional polarimeters are being replacing by polarimeters using light of longer wavelengths, λ =880 nm, which can be used for monitoring a colored (through not a turbid) solution, and so decreases solid waste .Kinetics of Sucrose Hydrolysis (Inversion) Followed by Polarimetry Purposes: 1. To determine the rate constant of sucrose hydrolysis reaction by polarimetry method. 2. To study the effect of catalyst (H) on the rate of the hydrolysis reaction. Theory: Review the most basic theory ofreaction rates and rate laws. Inversion Of Sucrose (IS)ObjectiveThe purpose of this experiment is to determine the rate constants for the acid catalyzed hydrolysis of sucrose at three tem.
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant . Sugar Inversion. Sucrose is chemically unstable when dissolved in water. The disaccharide molecule breaks in half to form two monosaccharide units: one molecule of glucose and one molecule of fructose. . Because of this effect, polarimetry is a very convenient way to measure the rate of inversion or the degree of inversion in partially .
INVERSION OF SUCROSE Muhammad Sagir Abubakar . AP-300 Automatic Polarimeter, with brand name; ATAGO sold under conbest was used which is made of sodium lamp with λ=589nm. Polarimetric .
work instruction for rockwell hardness tester pdf
out the long polarimeter tube with DI water. Obtain 20g of sucrose and pour it into a 50mL volumetric flask fill with water up to the mark. Dissolve the sugar in about 40 mL of water and shake vigorously. Transfer 5 mL of this sucrose solution into a 25 mL, 50 mL and 100 mL volumetric flask. Starting with the least concentrated solution (100 mL flask solution), transfer .Polarimetry is the measurement of optical rotation of substances by using a polarimeter. A polarimeter is an instrument which measures the angle of rotation by passing polarized light through an optically active (chiral) substance. To measure optical rotation, a Light Emitting Diode (LED) produces a beam of ordinary light.
After emptying out the sucrose solution from the polarimeter tube, a second mixture was created using a second 25 mL aliquot of sucrose solution and a 25 mL volume of 4M hydrochloric acid. . The rate law for the inversion of sucrose, in the presence of a large excess of acid, is of the form: δ[Sucrose] δt = keff [sucrose] 1. In tracking the .
In case of inversion of cane sugar the rate of reaction depends on the concentrations of sucrose, water and of hydrogen ion acts as a catalyst. The concentration of water may be regarded as constant when a large excess is used and H+ ion remains constant during the reaction. Under these conditions the reaction is first order in sucrose.For the inversion of sucrose catalysed by hydrogen ions sucrose 4- H+ + H20 -> X glucose+fructose + H+, formula (2-1, becomes b2 = W M fz. (2-3) where S denotes sucrose. The reacting complex X has a single positive charge the same as the hydrogen ion. Consequently, when we use (2*2), the terms proportional to Az\ cancel and we are left with
webSSG 08 | Fever Dream. Restricted Sniper Rifle. StatTrak Available. Inspect (FN) Inspect (MW) Inspect (FT) Inspect (WW) Inspect (BS) Prisma 2 Case. The Prisma 2 Collection. 3736 Steam Market Listings. StatTrak Factory New $7.36. StatTrak Minimal Wear $3.26.
inversion of sucrose by polarimeter|sucrose inversion research paper